The Periodic Table: An Organized Universe of Elements
Periodic Table elements – Picture a vast chart meticulously categorizing every known chemical element based on its atomic number and electron configuration. This is precisely what the periodic table accomplishes. The table comprises rows and columns, where rows are referred to as periods, and columns are known as groups. Dmitri Mendeleev, a pioneering Russian chemist, played a pivotal role in its development in 1869. This article delves into the essential concepts of the periodic table, shedding light on periods, groups, and the enduring legacy of Mendeleev’s contribution to the field of chemistry.
Periods: Horizontal Rows of Elemental Evolution
Within the periodic table, periods are represented as horizontal rows that extend from left to right. There are seven periods, numbered from 1 to 7, located on the left side of the table. As you traverse a period from left to right, the atomic numbers of the elements progressively increase. This ascending atomic number signifies the addition of protons in the nucleus, resulting in an increase in electron shells or energy levels. Consequently, the electron configuration of elements undergoes changes within a period.
It’s essential to note that elements within the same period may exhibit vastly different chemical properties. Despite their proximity in the table, the atomic number and electron configuration play a crucial role in determining an element’s unique characteristics. In other words, elements in a period are not necessarily chemically similar.
Groups: Vertical Columns of Elemental Kin
Groups, also known as families, form the vertical columns of the periodic table. There are a total of 18 numbered groups in the table, although it’s worth mentioning that the f-block columns, nestled between groups 2 and 3, do not bear numerical designations. Each group consists of elements that share similar chemical properties, owing to their analogous electron configurations in the outermost electron shell.
The group number signifies the number of valence electrons, which are the electrons in the outermost energy level, shared by all elements within that group. These valence electrons heavily influence an element’s chemical behavior, making elements within the same group chemically related. Consequently, elements belonging to the same group often exhibit comparable reactivity, bonding tendencies, and general chemical behavior.
Dmitri Mendeleev’s Ingenious Contributions
The development of the periodic table wouldn’t be complete without acknowledging the pioneering work of Dmitri Mendeleev. In 1869, Mendeleev revolutionized the world of chemistry by arranging elements based on their properties. Remarkably, he left gaps in the table for elements that had not yet been discovered, predicting their properties with uncanny accuracy. His foresight in creating a table with empty slots for future discoveries underscores the depth of his understanding of the periodic trends of elements.
In conclusion, the periodic table is more than just a chart; it’s a roadmap that guides chemists in their exploration of the fundamental building blocks of matter. Periods and groups provide a structured framework for understanding the behavior of elements, while Dmitri Mendeleev’s visionary contributions continue to inspire scientific discovery to this day. As we peer into this organized universe of elements, we uncover the remarkable patterns and properties that define the world of chemistry.
Periodic is an arrangement of elements, ordered by their atomic number and electron configuration. In the table rows are called as periods and columns are called groups. The Russian chemist Dmitri Mendeleev develops the periodic table based on their properties in 1869.
Please see the below periodic table.
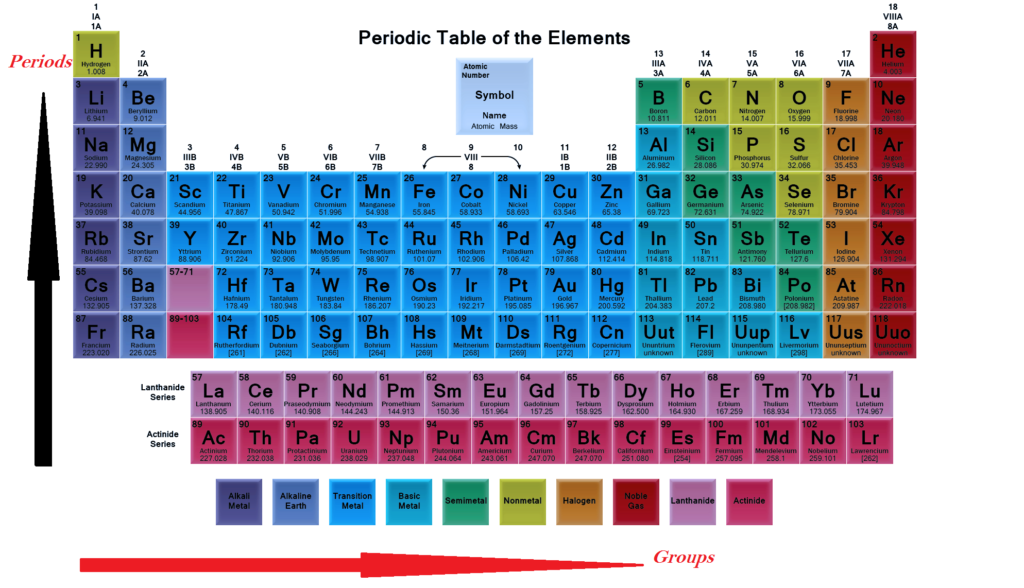
Please see the below periodic table elements. Periods in the periodic table elements. In each period (horizontal row), the atomic numbers increase from left to right. The periods are numbered 1 through 7 on the left-hand side of the table. Elements that are in the same period have chemical properties that are not all that similar.
A group (also known as a family) is a column of elements in the Periodic table (periodic table elements) of the chemical elements. There are 18 numbered groups in the periodic table, but the f-block columns (between groups 2 and 3) are not numbered.
Periodic Table elements with Electron Affinity:
List of Elements:
This table has electron affinity of Periodic table elements, symbol and discovered year.
| Sno | Name | Symbol | Discover Year | Group | Electron configuration |
|---|---|---|---|---|---|
| 1 | Hydrogen | H | 1776 | 1 | 1s1 |
| 2 | Helium | He | 1895 | 18 | 1s2 |
| 3 | Lithium | Li | 1817 | 1 | [He] 2s1 |
| 4 | Beryllium | Be | 1797 | 2 | [He] 2s2 |
| 5 | Boron | B | 1808 | 13 | [He] 2s2 2p1 |
| 6 | Carbon | C | ancient | 14 | [He] 2s2 2p2 |
| 7 | Nitrogen | N | 1772 | 15 | [He] 2s2 2p3 |
| 8 | Oxygen | O | 1774 | 16 | [He] 2s2 2p4 |
| 9 | Fluorine | F | 1886 | 17 | [He] 2s2 2p5 |
| 10 | Neon | Ne | 1898 | 18 | [He] 2s2 2p6 |
| 11 | Sodium | Na | 1807 | 1 | [Ne] 3s1 |
| 12 | Magnesium | Mg | 1755 | 2 | [Ne] 3s2 |
| 13 | Aluminum | Al | 1825 | 13 | [Ne] 3s2 3p1 |
| 14 | Silicon | Si | 1824 | 14 | [Ne] 3s2 3p2 |
| 15 | Phosphorus | P | 1669 | 15 | [Ne] 3s2 3p3 |
| 16 | Sulfur | S | ancient | 16 | [Ne] 3s2 3p4 |
| 17 | Chlorine | Cl | 1774 | 17 | [Ne] 3s2 3p5 |
| 18 | Argon | Ar | 1894 | 18 | [Ne] 3s2 3p6 |
| 19 | Potassium | K | 1807 | 1 | [Ar] 4s1 |
| 20 | Calcium | Ca | 1808 | 2 | [Ar] 4s2 |
| 21 | Scandium | Sc | 1879 | 3 | [Ar] 3d1 4s2 |
| 22 | Titanium | Ti | 1791 | 4 | [Ar] 3d2 4s2 |
| 23 | Vanadium | V | 1830 | 5 | [Ar] 3d3 4s2 |
| 24 | Chromium | Cr | 1797 | 6 | [Ar] 3d5 4s1 |
| 25 | Manganese | Mn | 1774 | 7 | [Ar] 3d5 4s2 |
| 26 | Iron | Fe | ancient | 8 | [Ar] 3d6 4s2 |
| 27 | Cobalt | Co | 1735 | 9 | [Ar] 3d7 4s2 |
| 28 | Nickel | Ni | 1751 | 10 | [Ar] 3d8 4s2 |
| 29 | Copper | Cu | ancient | 11 | [Ar] 3d10 4s1 |
| 30 | Zinc | Zn | ancient | 12 | [Ar] 3d10 4s2 |
| 31 | Gallium | Ga | 1875 | 13 | [Ar] 3d10 4s2 4p1 |
| 32 | Germanium | Ge | 1886 | 14 | [Ar] 3d10 4s2 4p2 |
| 33 | Arsenic | As | ancient | 15 | [Ar] 3d10 4s2 4p3 |
| 34 | Selenium | Se | 1817 | 16 | [Ar] 3d10 4s2 4p4 |
| 35 | Bromine | Br | 1826 | 17 | [Ar] 3d10 4s2 4p5 |
| 36 | Krypton | Kr | 1898 | 18 | [Ar] 3d10 4s2 4p6 |
| 37 | Rubidium | Rb | 1861 | 1 | [Kr] 5s1 |
| 38 | Strontium | Sr | 1790 | 2 | [Kr] 5s2 |
| 39 | Yttrium | Y | 1794 | 3 | [Kr] 4d1 5s2 |
| 40 | Zirconium | Zr | 1789 | 4 | [Kr] 4d2 5s2 |
| 41 | Niobium | Nb | 1801 | 5 | [Kr] 4d4 5s1 |
| 42 | Molybdenum | Mo | 1781 | 6 | [Kr] 4d5 5s1 |
| 43 | Technetium | Tc | 1937 | 7 | [Kr] 4d5 5s2 |
| 44 | Ruthenium | Ru | 1844 | 8 | [Kr] 4d7 5s1 |
| 45 | Rhodium | Rh | 1803 | 9 | [Kr] 4d8 5s1 |
| 46 | Palladium | Pd | 1803 | 10 | [Kr] 4d10 |
| 47 | Silver | Ag | ancient | 11 | [Kr] 4d10 5s1 |
| 48 | Cadmium | Cd | 1817 | 12 | [Kr] 4d10 5s2 |
| 49 | Indium | In | 1863 | 13 | [Kr] 4d10 5s2 5p1 |
| 50 | Tin | Sn | ancient | 14 | [Kr] 4d10 5s2 5p2 |
| 51 | Antimony | Sb | ancient | 15 | [Kr] 4d10 5s2 5p3 |
| 52 | Tellurium | Te | 1783 | 16 | [Kr] 4d10 5s2 5p4 |
| 53 | Iodine | I | 1811 | 17 | [Kr] 4d10 5s2 5p5 |
| 54 | Xenon | Xe | 1898 | 18 | [Kr] 4d10 5s2 5p6 |
| 55 | Cesium | Cs | 1860 | 1 | [Xe] 6s1 |
| 56 | Barium | Ba | 1808 | 2 | [Xe] 6s2 |
| 57 | Lanthanum | La | 1839 | 3 | [Xe] 5d1 6s2 |
| 58 | Cerium | Ce | 1803 | 101 | [Xe] 4f1 5d1 6s2 |
| 59 | Praseodymium | Pr | 1885 | 101 | [Xe] 4f3 6s2 |
| 60 | Neodymium | Nd | 1885 | 101 | [Xe] 4f4 6s2 |
| 61 | Promethium | Pm | 1945 | 101 | [Xe] 4f5 6s2 |
| 62 | Samarium | Sm | 1879 | 101 | [Xe] 4f6 6s2 |
| 63 | Europium | Eu | 1901 | 101 | [Xe] 4f7 6s2 |
| 64 | Gadolinium | Gd | 1880 | 101 | [Xe] 4f7 5d1 6s2 |
| 65 | Terbium | Tb | 1843 | 101 | [Xe] 4f9 6s2 |
| 66 | Dysprosium | Dy | 1886 | 101 | [Xe] 4f10 6s2 |
| 67 | Holmium | Ho | 1867 | 101 | [Xe] 4f11 6s2 |
| 68 | Erbium | Er | 1842 | 101 | [Xe] 4f12 6s2 |
| 69 | Thulium | Tm | 1879 | 101 | [Xe] 4f13 6s2 |
| 70 | Ytterbium | Yb | 1878 | 101 | [Xe] 4f14 6s2 |
| 71 | Lutetium | Lu | 1907 | 101 | [Xe] 4f14 5d1 6s2 |
| 72 | Hafnium | Hf | 1923 | 4 | [Xe] 4f14 5d2 6s2 |
| 73 | Tantalum | Ta | 1802 | 5 | [Xe] 4f14 5d3 6s2 |
| 74 | Tungsten | W | 1783 | 6 | [Xe] 4f14 5d4 6s2 |
| 75 | Rhenium | Re | 1925 | 7 | [Xe] 4f14 5d5 6s2 |
| 76 | Osmium | Os | 1803 | 8 | [Xe] 4f14 5d6 6s2 |
| 77 | Iridium | Ir | 1803 | 9 | [Xe] 4f14 5d7 6s2 |
| 78 | Platinum | Pt | 1735 | 10 | [Xe] 4f14 5d9 6s1 |
| 79 | Gold | Au | ancient | 11 | [Xe] 4f14 5d10 6s1 |
| 80 | Mercury | Hg | ancient | 12 | [Xe] 4f14 5d10 6s2 |
| 81 | Thallium | Tl | 1861 | 13 | [Xe] 4f14 5d10 6s2 6p1 |
| 82 | Lead | Pb | ancient | 14 | [Xe] 4f14 5d10 6s2 6p2 |
| 83 | Bismuth | Bi | ancient | 15 | [Xe] 4f14 5d10 6s2 6p3 |
| 84 | Polonium | Po | 1898 | 16 | [Xe] 4f14 5d10 6s2 6p4 |
| 85 | Astatine | At | 1940 | 17 | [Xe] 4f14 5d10 6s2 6p5 |
| 86 | Radon | Rn | 1900 | 18 | [Xe] 4f14 5d10 6s2 6p6 |
| 87 | Francium | Fr | 1939 | 1 | [Rn] 7s1 |
| 88 | Radium | Ra | 1898 | 2 | [Rn] 7s2 |
| 89 | Actinium | Ac | 1899 | 3 | [Rn] 6d1 7s2 |
| 90 | Thorium | Th | 1829 | 102 | [Rn] 6d2 7s2 |
| 91 | Protactinium | Pa | 1913 | 102 | [Rn] 5f2 6d1 7s2 |
| 92 | Uranium | U | 1789 | 102 | [Rn] 5f3 6d1 7s2 |
| 93 | Neptunium | Np | 1940 | 102 | [Rn] 5f4 6d1 7s2 |
| 94 | Plutonium | Pu | 1940 | 102 | [Rn] 5f6 7s2 |
| 95 | Americium | Am | 1944 | 102 | [Rn] 5f7 7s2 |
| 96 | Curium | Cm | 1944 | 102 | |
| 97 | Berkelium | Bk | 1949 | 102 | |
| 98 | Californium | Cf | 1950 | 102 | |
| 99 | Einsteinium | Es | 1952 | 102 | |
| 100 | Fermium | Fm | 1952 | 102 | |
| 101 | Mendelevium | Md | 1955 | 102 | |
| 102 | Nobelium | No | 1958 | 102 | |
| 103 | Lawrencium | Lr | 1961 | 102 | |
| 104 | Rutherfordium | Rf | 1964 | 4 | |
| 105 | Dubnium | Db | 1967 | 5 | |
| 106 | Seaborgium | Sg | 1974 | 6 | |
| 107 | Bohrium | Bh | 1981 | 7 | |
| 108 | Hassium | Hs | 1984 | 8 | |
| 109 | Meitnerium | Mt | 1982 | 9 |
Periodic Table elements wikipedia is here, read each element properties here

